|
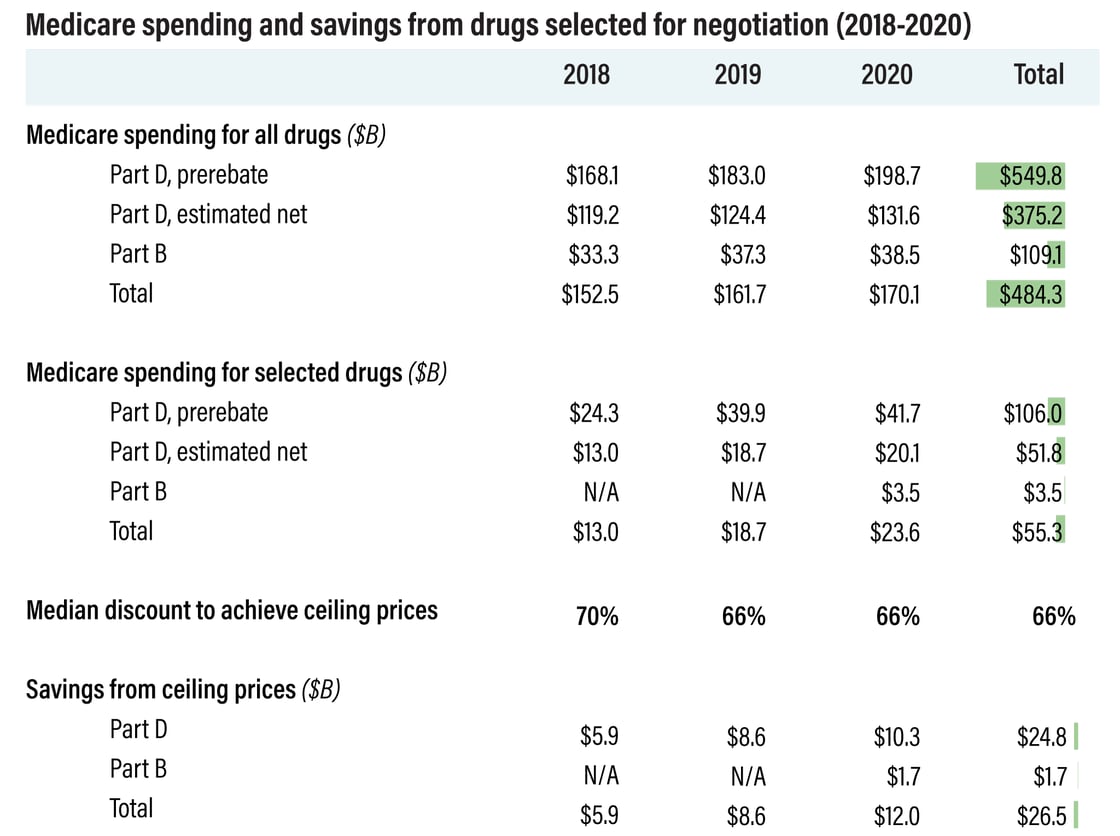
The study authors
pointed out that such a stringent selection process will hamper savings
potential. Drugs must be chosen in order of highest spending, which could
create missed opportunities if costlier therapies get generic competition
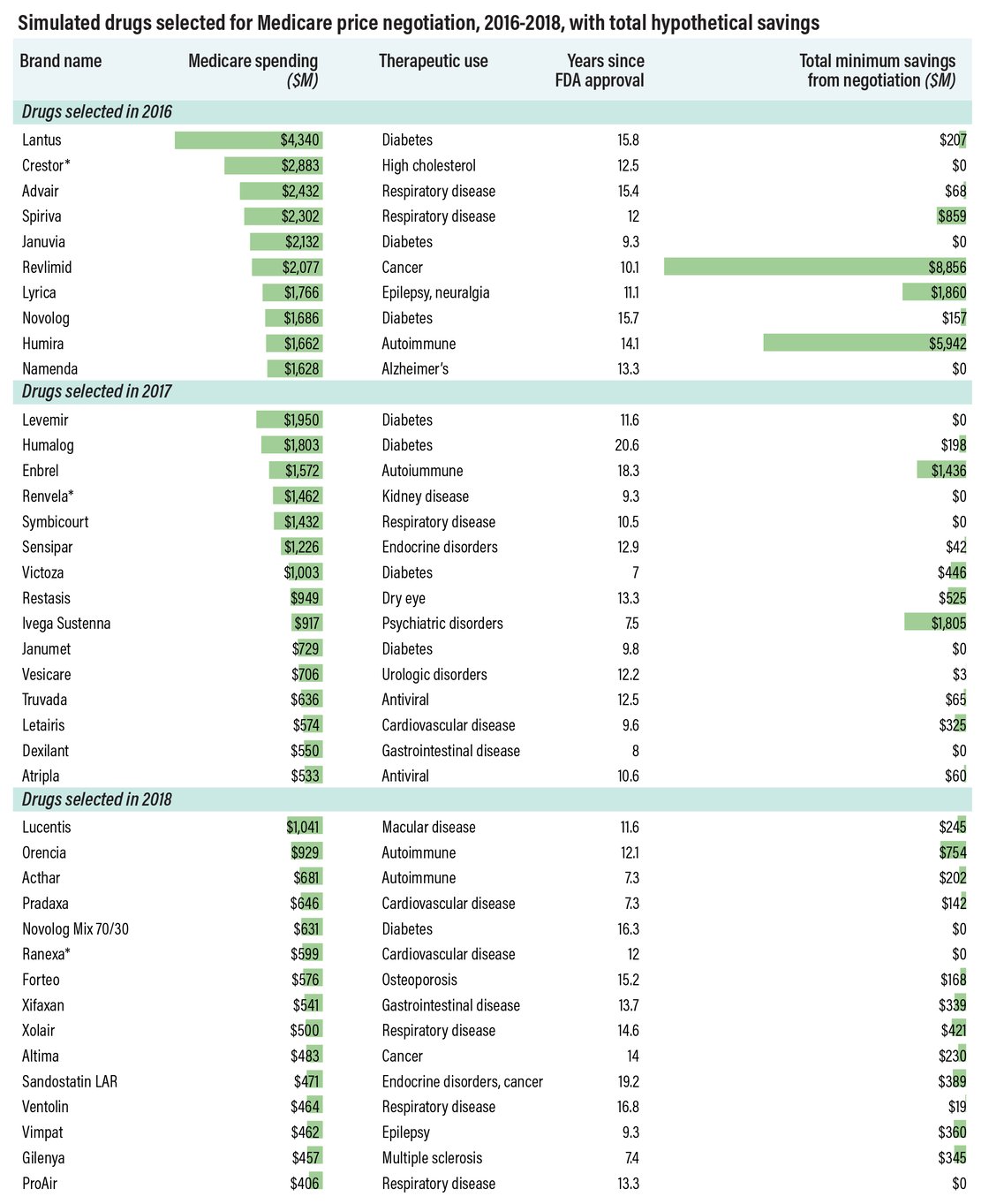
before a negotiated price goes into effect. This happened with three
drugs in the simulation: Crestor, Renvela and Ranexa (see chart, below).
Meanwhile, drugs like AbbVie’s Humira and Pfizer’s Lyrica generated some
of the greatest savings ($5.9 billion and $1.9 billion, respectively) in
the simulation, but they won’t be eligible for price negotiation in the
real world. That’s because Lyrica’s first generics launched in 2019, while Humira’s first biosimilar,
Amgen Inc.’s Amjevita, launched last month, with more to follow.
The study authors also
noted that negotiation could create “unanticipated market effects” if CMS
selects one drug in a particular class, but not another. Manufacturers
could feel pressured to match government-negotiated prices, but they
could also leverage payer rebates to maintain higher prices. This “could
create situations in which negotiated rebates for non-selected drugs
encourage Part D plans to offer a preferred formulary position for
non-selected competitors carrying higher prices for patients,” the
authors wrote. Drugmakers could also launch new formulations of existing
products to avoid selection.
|
No comments:
Post a Comment