|
The FDA in May
approved Radicava ORS (edaravone), an orally administered version of
Mitsubishi Tanabe Pharma America’s Radicava, for the treatment of adults
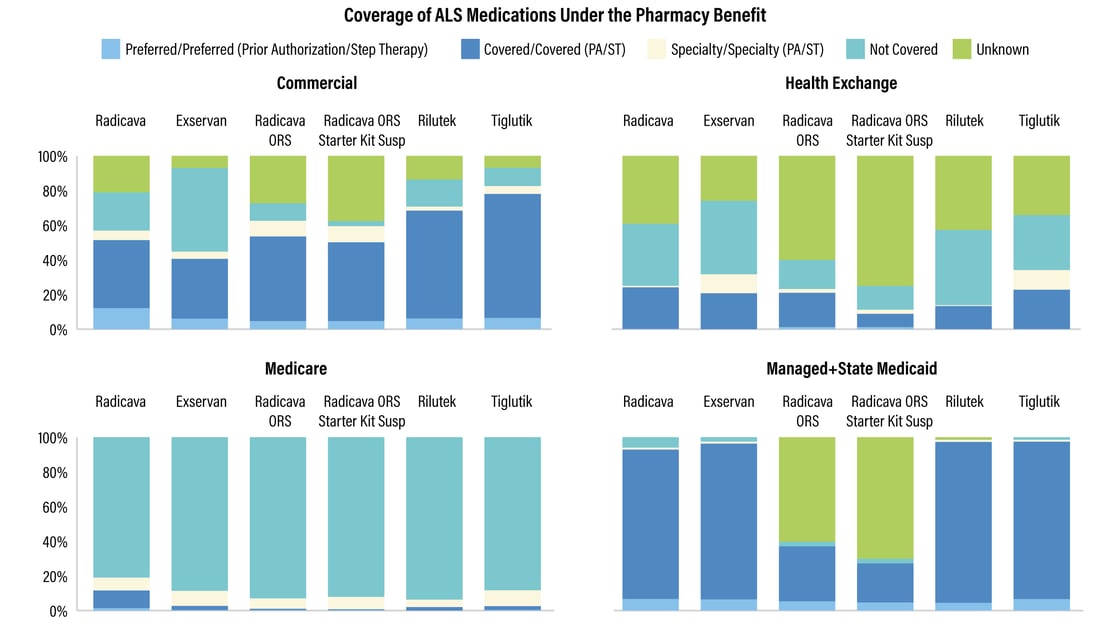
with amyotrophic lateral sclerosis (ALS). The six agents available to
treat ALS are primarily covered under the pharmacy benefit, with medical
benefit coverage available for only Radicava. For most ALS medications,
more than half of commercial covered lives are under the preferred
tier/preferred with prior authorization or step therapy and covered
tier/covered with PA/ST.
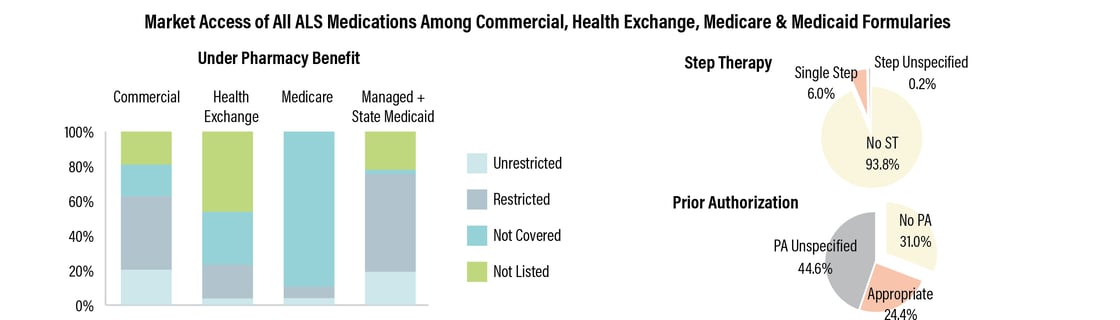
Under the pharmacy
benefit, about 42% of lives under commercial formularies have coverage
for ALS drugs with utilization management restrictions. Meanwhile, almost
90% of lives under Medicare formularies lack coverage for at least one
ALS therapy. For about 69% of covered lives, payer-controlled pharmacy
benefit formularies require prior authorization.
|
No comments:
Post a Comment